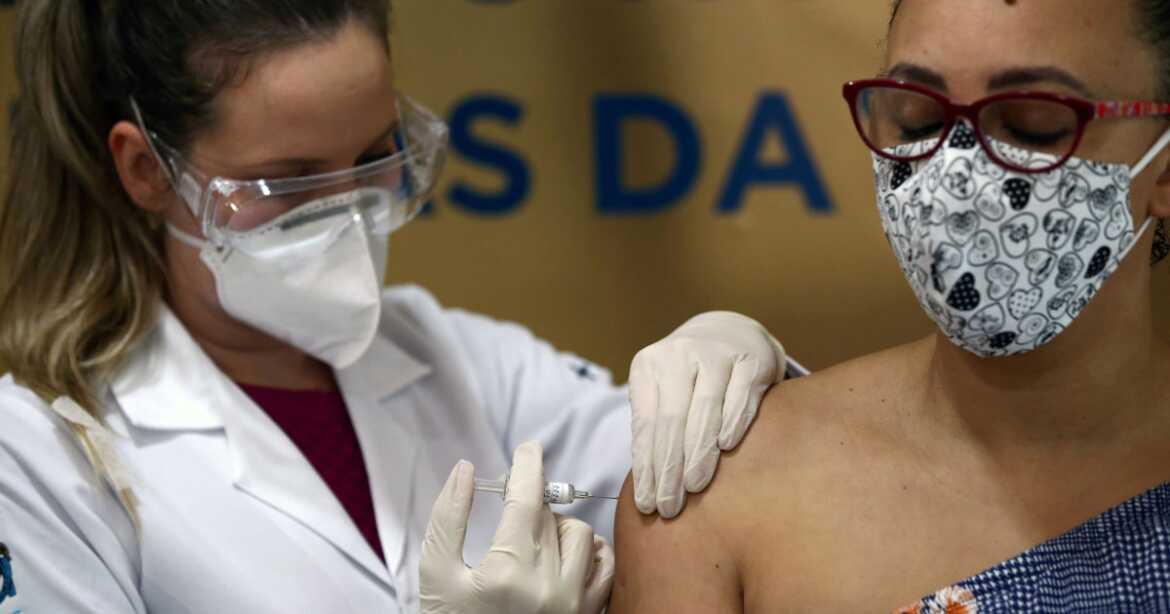
Brazil recently approved CoronaVac, the vaccine made by Beijing-based company Sinovac, thereby enabling Latin America’s largest nation to begin their immunization program. CoronaVac has demonstrated 100% protection against severe COVID-19 and hospitalization in the recently-concluded clinical trial involving 12,476 healthcare workers in Brazil. This trial is currently the only publicly disclosed phase 3 clinical trial (involving COVID-19 vaccines), where participants were all healthcare workers working in hospitals. Accordingly, it provides an actual indication of the capability of this vaccine to protect the critical healthcare workforce.
Several days after Brazil’s announcement, Chile announced the emergency use approval of CoronaVac as well. Sinovac has agreements in place with at least eight countries, including Turkey, Singapore, Indonesia, Thailand, the Philippines, Malaysia, Chile, and Brazil.
Brazil’s National Health Surveillance Agency began rolling out CoronaVac on Monday, and has announced plans to vaccinate priority groups starting with health workers, people above 60 years of age, disabled people, and the indigenous population. The country now has six million doses of Sinovac’s CoronaVac on hand.
Previously, the 50.4% overall efficacy reported from Brazil’s Phase 3 trials drew public attention. Investigators, however, soon pointed out that the results came from all cases, including “very mild” cases that did not require medical intervention. It was further revealed that the study participants were all healthcare workers constantly exposed to COVID-19 transmissions in the hospital setting, at attack rates close to five times the average.
“Vaccine efficacy must be thoroughly evaluated in relation to the methodology. In the case of the Brazil trial, their analysis included very mild COVID-19 cases occurring in high transmission-risk settings,” noted Dr. Noel Miranda, a member of the scientific team of IP Biotech, Inc., a Filipino biotech/vaccine provider.
“The overall protection rate of 50.4%, given the circumstances, is indeed acceptable and provides a sense of assurance in light of the 100% efficacy in preventing COVID-19 hospitalization obtained in Brazil and Turkey,” he added. “Given the current understanding that none of the EUA-approved vaccines are capable of preventing asymptomatic and mild infections, the true merit of being vaccinated lies in reducing disease severity and mortality, where the disease would just manifest as a common cold with mild symptoms. By aiming for this, we can also help alleviate fear.” he further explained.
CoronaVac was recently granted approval by the Food and Drug Administration of the Philippines (FDA) and the Department of Health’s Single Joint Research Ethics Board (SJREB) to conduct clinical trials in the Philippines. Sponsored and financed by IP Biotech, Inc., the study will be a randomized, double-blind, placebo-controlled clinical trial conducted on volunteers aged 60 to 80 years.
“The trial, which is representative of our local circumstances and in a target age group, will provide supplemental data to the global trials,” Miranda went on to say. “Given the FDA approval and the unbiased framework of the study, I am optimistic that CoronaVac’s worthiness for massive vaccination will further be validated. It is our hope that the Filipinos, especially our elderly population, would all benefit from the protection that this promising vaccine offers,” he concluded.
